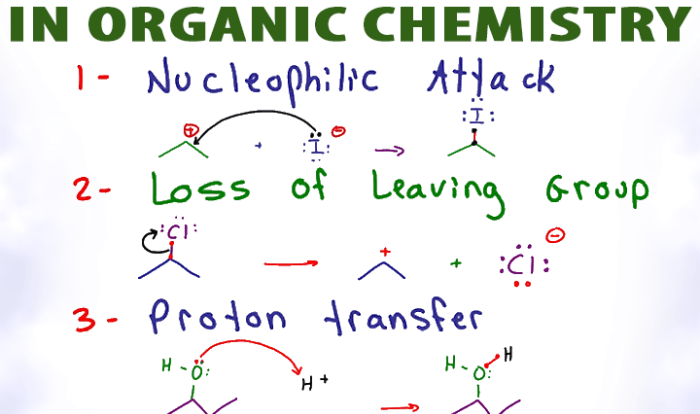
Axmen classification for benzene c6h6 – In the realm of organic chemistry, the AXMEN classification system provides a powerful tool for understanding the intricate molecular structure and reactivity of benzene (C6H6). This classification system, a cornerstone of chemistry, has revolutionized our comprehension of aromatic compounds, paving the way for groundbreaking advancements in various scientific disciplines.
AXMEN, an acronym for “Adjacent to X, Meta to X, and ortho to X, Neighboring to X, and Meta to X,” offers a systematic approach to classifying the relative positions of substituents on a benzene ring. This classification system not only aids in predicting the reactivity of benzene derivatives but also plays a crucial role in comprehending their physical and chemical properties.
Benzene C6H6 Properties
Benzene is a colorless, flammable liquid with a characteristic odor. It is a hydrocarbon with the chemical formula C6H6, consisting of a six-membered ring of carbon atoms with alternating single and double bonds.Benzene has a boiling point of 80.1 °C, a melting point of 5.5 °C, and a density of 0.879 g/mL.
It is insoluble in water but soluble in organic solvents such as ethanol and diethyl ether.
Benzene C6H6 Reactivity: Axmen Classification For Benzene C6h6
Benzene is a highly reactive compound that undergoes a variety of reactions, including electrophilic aromatic substitution, addition, and oxidation.Electrophilic aromatic substitution is the most common reaction of benzene. In this reaction, an electrophile (a species that is attracted to electrons) attacks the benzene ring, forming a new bond between the electrophile and one of the carbon atoms in the ring.
The catalyst for this reaction is typically a Lewis acid, such as aluminum chloride.Examples of electrophilic aromatic substitution reactions include:
- Nitration: The reaction of benzene with nitric acid and sulfuric acid to form nitrobenzene.
- Halogenation: The reaction of benzene with chlorine or bromine to form chlorobenzene or bromobenzene.
- Alkylation: The reaction of benzene with an alkyl halide to form an alkylbenzene.
Benzene C6H6 Applications
Benzene is a major industrial chemical that is used in the production of a variety of products, including:
- Styrene: A monomer used in the production of polystyrene, a plastic used in a variety of applications, including food packaging, disposable cups, and toys.
- Phenol: A compound used in the production of plastics, resins, and dyes.
- Ethylbenzene: A solvent used in the production of paints, varnishes, and adhesives.
Benzene is also used as a fuel and as a component of gasoline. However, due to its toxicity, the use of benzene in gasoline has been phased out in many countries.
Benzene C6H6 Spectroscopy
Benzene can be identified and characterized using a variety of spectroscopic techniques, including UV-Vis spectroscopy and NMR spectroscopy.UV-Vis spectroscopy measures the absorption of ultraviolet and visible light by a compound. The UV-Vis spectrum of benzene shows a characteristic absorption band at 254 nm, which is due to the π-π* transition of the benzene ring.NMR
spectroscopy measures the absorption of radio waves by the nuclei of atoms in a compound. The NMR spectrum of benzene shows a characteristic singlet at 7.3 ppm, which is due to the protons on the benzene ring.
Benzene C6H6 Derivatives
Benzene can be modified by the addition of various functional groups to form a variety of derivatives. Some of the most common benzene derivatives include:
- Toluene: A methylbenzene that is used as a solvent and in the production of other chemicals.
- Xylene: A dimethylbenzene that is used as a solvent and in the production of other chemicals.
- Aniline: An aminobenzene that is used in the production of dyes and other chemicals.
Benzene derivatives have a wide range of applications, including in the production of plastics, dyes, pharmaceuticals, and pesticides.
Essential Questionnaire
What is the significance of the AXMEN classification system for benzene C6H6?
The AXMEN classification system provides a systematic approach to understanding the relative positions of substituents on a benzene ring, aiding in the prediction of reactivity and comprehension of physical and chemical properties.
How does the AXMEN classification system help predict the reactivity of benzene derivatives?
The AXMEN classification system allows chemists to identify the relative positions of substituents on a benzene ring, which influences the electronic properties of the molecule and, consequently, its reactivity.
What are the practical applications of the AXMEN classification system in chemistry?
The AXMEN classification system finds applications in various chemistry disciplines, including organic synthesis, medicinal chemistry, and materials science, where it guides the design and development of new compounds with specific properties.