Gizmo boyle’s law and charles law – Gizmo Boyle’s Law and Charles’ Law, cornerstones of gas behavior, unveil the intricate relationship between pressure, volume, and temperature. These laws govern the behavior of gases under varying conditions, providing a fundamental understanding of gas dynamics. Join us as we delve into the fascinating world of Boyle’s Law and Charles’ Law, exploring their applications and implications in science and engineering.
Boyle’s Law illuminates the inverse relationship between pressure and volume at constant temperature. As pressure increases, volume decreases, and vice versa. This principle finds practical applications in scuba diving, where Boyle’s Law explains the changes in gas volume experienced by divers at different depths.
Boyle’s Law
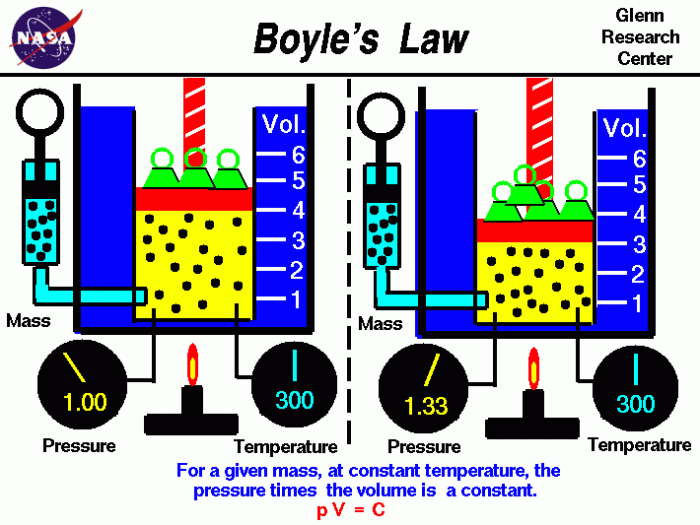
Boyle’s Law describes the inverse relationship between the pressure and volume of a gas at constant temperature. As the pressure of a gas increases, its volume decreases proportionally, and vice versa. This relationship can be expressed mathematically as P1V1 = P2V2, where P1 and V1 represent the initial pressure and volume, and P2 and V2 represent the final pressure and volume.
Real-world examples of Boyle’s Law include the behavior of air in a bicycle pump and the compression of gases in engines. In a bicycle pump, as the piston is pushed down, the volume of the air inside the pump decreases, causing the pressure to increase.
Similarly, in an engine, the compression of the air-fuel mixture before ignition increases its pressure and temperature.
Boyle’s Law has limitations, however. It only applies to ideal gases and assumes constant temperature. In real gases, deviations from ideal behavior can occur at high pressures or low temperatures.
Charles’s Law
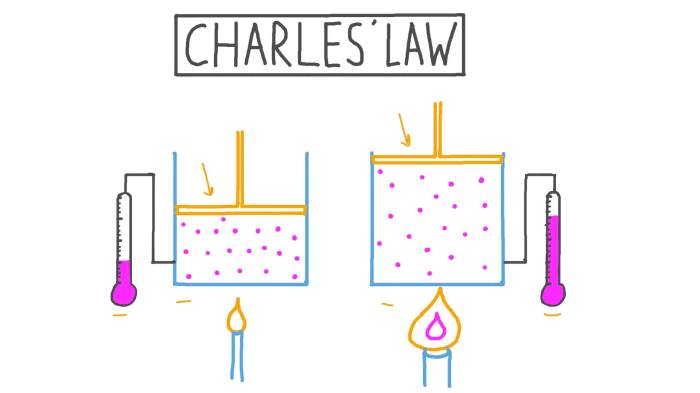
Charles’s Law describes the direct relationship between the temperature and volume of a gas at constant pressure. As the temperature of a gas increases, its volume increases proportionally, and vice versa. This relationship can be expressed mathematically as V1/T1 = V2/T2, where V1 and T1 represent the initial volume and temperature, and V2 and T2 represent the final volume and temperature.
Real-world examples of Charles’s Law include the expansion of air in a hot air balloon and the cooling of gases in refrigerators. In a hot air balloon, as the air inside the balloon is heated, its volume increases, causing the balloon to rise.
Similarly, in a refrigerator, the cooling of the air inside the fridge causes its volume to decrease, resulting in air circulation.
Charles’s Law also has limitations. It only applies to ideal gases and assumes constant pressure. In real gases, deviations from ideal behavior can occur at high temperatures or low pressures.
Comparison of Boyle’s Law and Charles’s Law: Gizmo Boyle’s Law And Charles Law
| Feature | Boyle’s Law | Charles’s Law |
|---|---|---|
| Relationship | Inverse relationship between pressure and volume (at constant temperature) | Direct relationship between temperature and volume (at constant pressure) |
| Mathematical Equation | P1V1 = P2V2 | V1/T1 = V2/T2 |
| Real-World Examples | Bicycle pump, engine compression | Hot air balloon, refrigerator cooling |
| Limitations | Only applies to ideal gases, assumes constant temperature | Only applies to ideal gases, assumes constant pressure |
Similarities between Boyle’s Law and Charles’s Law include their applicability to ideal gases and their mathematical forms. Both laws can be used to solve problems involving changes in pressure, volume, and temperature.
Differences between Boyle’s Law and Charles’s Law lie in the variables they relate. Boyle’s Law focuses on the inverse relationship between pressure and volume, while Charles’s Law focuses on the direct relationship between temperature and volume.
Applications of Boyle’s Law and Charles’s Law
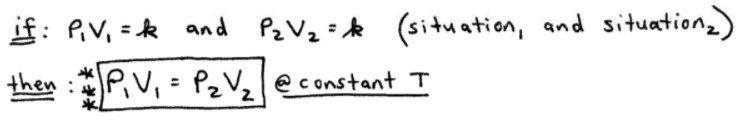
Boyle’s Law and Charles’s Law have numerous applications in everyday life, science, and engineering.
- Scuba diving:Boyle’s Law explains the increased pressure on a diver as they descend deeper into the water. This pressure can cause nitrogen to dissolve in the diver’s blood, leading to decompression sickness if not properly managed.
- Weather forecasting:Charles’s Law is used to predict changes in atmospheric pressure and temperature. By measuring the volume of a gas at different temperatures, meteorologists can determine the temperature at higher altitudes.
- Engineering:Boyle’s Law is used in the design of pressure vessels and pipelines. Engineers need to ensure that these vessels can withstand the changes in pressure that occur during operation.
- Medicine:Charles’s Law is used in the design of medical equipment, such as ventilators and anesthesia machines. These devices need to deliver gases at specific volumes and temperatures to ensure proper patient care.
Boyle’s Law and Charles’s Law continue to be important tools for understanding the behavior of gases in various applications. Their principles are essential for advancements in fields such as engineering, medicine, and environmental science.
FAQ
What is Boyle’s Law?
Boyle’s Law describes the inverse relationship between the pressure and volume of a gas at constant temperature.
How is Charles’ Law different from Boyle’s Law?
Charles’ Law describes the direct relationship between the volume and temperature of a gas at constant pressure, while Boyle’s Law focuses on pressure and volume.
What are some real-world applications of Boyle’s Law?
Boyle’s Law finds applications in scuba diving, where it explains the changes in gas volume experienced by divers at different depths.