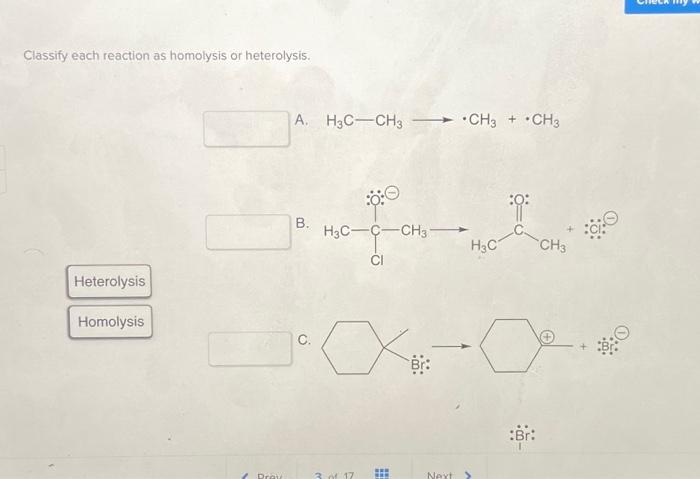
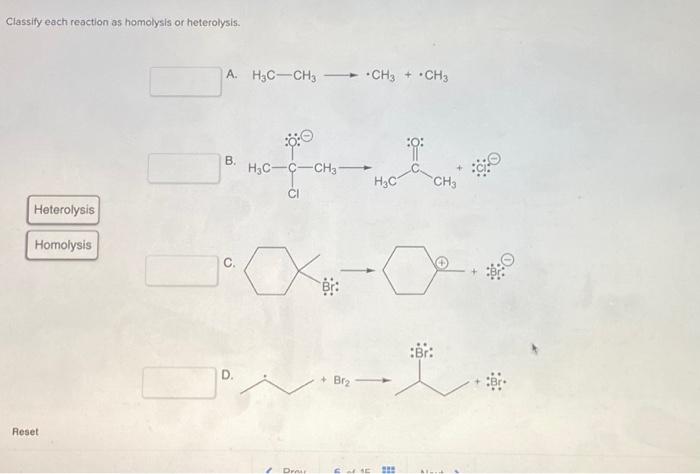
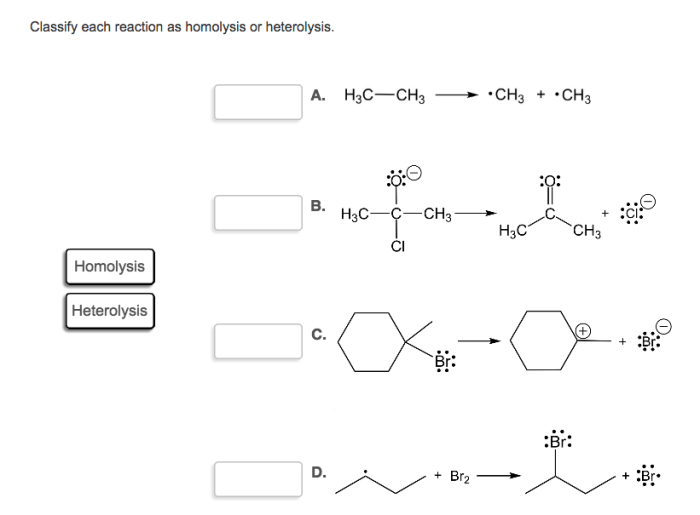
Classify each reaction as homolysis or heterolysis. – Delving into the realm of chemical bond cleavage, we embark on an exploration of homolysis and heterolysis. These distinct mechanisms play a pivotal role in determining the course of chemical reactions, shaping the products formed and the pathways taken. Understanding their nuances is crucial for unraveling the intricacies of chemical transformations.
Homolysis, characterized by the symmetrical breaking of a covalent bond, contrasts with heterolysis, where the bond breaks asymmetrically, resulting in the formation of ions. This fundamental distinction governs the reactivity and selectivity of chemical species, influencing a wide range of processes in chemistry and beyond.
Definition of Homolysis and Heterolysis: Classify Each Reaction As Homolysis Or Heterolysis.
Bond cleavage is a fundamental process in chemistry that involves the breaking of chemical bonds. There are two main types of bond cleavage: homolysis and heterolysis.
Homolysisis the cleavage of a covalent bond in which each atom takes one of the electrons in the bond. This results in the formation of two free radicals, which are atoms or molecules with unpaired electrons. Heterolysis, on the other hand, is the cleavage of a covalent bond in which one atom takes both electrons in the bond.
This results in the formation of an ion and a neutral atom or molecule.
Examples of homolysis include the thermal decomposition of ethane and the photolysis of hydrogen chloride. Examples of heterolysis include the reaction of sodium metal with chlorine gas and the hydrolysis of water.
Mechanisms of Homolysis and Heterolysis
The mechanisms of homolysis and heterolysis are quite different. Homolysis typically occurs through a radical mechanism, in which the bond is broken by the homolytic cleavage of one of the electrons in the bond. This can occur through a variety of mechanisms, including thermal decomposition, photolysis, and radical chain reactions.
Heterolysis, on the other hand, typically occurs through an ionic mechanism, in which the bond is broken by the heterolytic cleavage of the electrons in the bond. This can occur through a variety of mechanisms, including nucleophilic attack, electrophilic attack, and acid-base reactions.
Factors Affecting Homolysis and Heterolysis
The type of bond cleavage that occurs is influenced by a number of factors, including the bond strength, the polarity of the bond, and the reaction conditions.
Bond strengthis a measure of the strength of the bond between two atoms. The stronger the bond, the more difficult it is to break. Homolysis is more likely to occur for weak bonds, while heterolysis is more likely to occur for strong bonds.
Bond polarityis a measure of the difference in electronegativity between two atoms. The more polar the bond, the more likely it is to undergo heterolysis. This is because the more electronegative atom will attract the electrons in the bond more strongly, leading to the formation of an ion and a neutral atom or molecule.
Reaction conditionscan also affect the type of bond cleavage that occurs. For example, high temperatures favor homolysis, while low temperatures favor heterolysis.
Consequences of Homolysis and Heterolysis
The consequences of homolysis and heterolysis are also quite different. Homolysis typically leads to the formation of free radicals, which are highly reactive species that can undergo a variety of reactions. These reactions can include radical chain reactions, which can lead to the formation of polymers and other complex molecules.
Heterolysis, on the other hand, typically leads to the formation of ions, which are less reactive than free radicals. Ions can undergo a variety of reactions, including acid-base reactions, precipitation reactions, and redox reactions.
Applications of Homolysis and Heterolysis, Classify each reaction as homolysis or heterolysis.
Homolysis and heterolysis are both important processes in chemistry. Homolysis is used in a variety of industrial processes, such as the production of plastics and pharmaceuticals. Heterolysis is used in a variety of biological processes, such as the metabolism of carbohydrates and the synthesis of proteins.
FAQ
What is the key difference between homolysis and heterolysis?
Homolysis involves the symmetrical breaking of a covalent bond, while heterolysis involves the asymmetrical breaking of a bond, resulting in the formation of ions.
What factors influence the type of bond cleavage that occurs?
Factors such as bond strength, polarity, and reaction conditions can influence the choice between homolysis and heterolysis.
What are the consequences of homolytic and heterolytic bond cleavage?
Homolytic cleavage can lead to the formation of free radicals, while heterolytic cleavage can lead to the formation of ions. These different products can have distinct reactivity and selectivity.